Abstract
Purpose Engineering bacteria can achieve targeted and controllable cancer therapy by utilizing synthetic biology technology and the characteristics of tumor microenvironment. Besides, the accurate tumor diagnosis and visualization of treatment process are also vital for bacterial therapy. In this study, lymphoma was used as a model. A series of experiments in vitro and in vivo were used to test the tumor targeting and accurate drug release ability of the drug system, to evaluate its visual diagnosis and antitumor effect, and to evaluate the safety of the live biotherapeutics and carrier system.
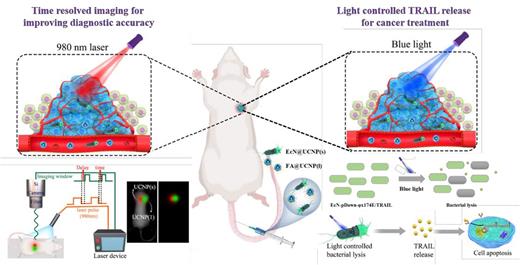
Results As shown in Figure 1, light control engineered bacteria system based on upconversion nanoparticles (UCNP) mediated time-resolved imaging (TRI) was constructed for lymphoma theranostic and therapy. The engineered bacteria were named as EcN-pDawn-φx174E/TRAIL, which lysed by blue light irradiation and then released tumor apoptosis-related inducing ligand (TRAIL). When the tumor was irradiated by blue light, TRAIL was released from bacteria to trigger tumor cell death. And then, UCNP with short luminous lifetime (UCNP(s)) were connected with anaerobic probiotics EcN-pDawn-φx174E/TRAIL to prepare EcN@UCNP(s), which targets tumor through the hypoxia targeting ability of EcN. UCNP with long luminous lifetime were modified with folic acid (FA) to prepare FA@UCNP, which targets tumor through the active targeting ability of FA. EcN@UCNP(s) and FA@UCNP were injected through tail vein, and both of them were co-located at tumor site. They can be distinguished with each other and used to precisely indicate the presence of tumor by TRI.
In vitro results showed that the released TRAIL from EcN could induce tumor cell death. In addition, the EcN-pDawn-φx174E/TRAIL+blue group showed the strongest therapeutic effect, which laid foundations for in vivo light control tumor treatment.
Subcutaneous Raji, U937 and Jurkat tumor model was constructed to evaluate the blue light control treatment effects. As indicated in bio-distribution studies, there were no bacteria accumulation in heart, spleen and lung tissues, the most bacteria were accumulated in tumor and they expanded over time. To explore whether the bacteria at the tumor site can function normally, the EcN-GFP were used for intuitive observation. The research demonstrated that GFP was expressed in tumor tissues, indicating that EcN-GFP@UCNP(s) targeted tumor, survived and expressed proteins. Besides, the TRAIL expression in tumor tissue was studied by ELISA assay, which showed that TRAIL could be normally expressed and released in therapy group.
The mice weight and tumor volume changes were measured every two days. There were no obvious changes in body weight of all mice. In addition, the blood routine and blood biochemical analysis were carried out after the experiment completed, and all indicators were within the normal range. And then HE staining of main organs in all groups explained the biosafety of these therapeutic materials
The tumor volume changes in each group were illustrated. In the blue light control tumor inhibition experiment, the therapy group displayed the smallest tumor volume (402 mm3), while the tumor volume in dark group was 1999 mm3, which was similar to that in control (2120 mm3) and FA@UCNP(l) group (2033 mm3). The therapy group revealed the highest tumor growth inhibition compared with other groups.
The changes of cytokine concentration in tumor tissues were also measured by ELISA assays. Obviously increased TNF-α, IFN-γ and IL-6 level were observed in therapy group. Besides, more tumor apoptosis was observed in therapy group. All these findings indicated that EcN-pDawn-φX174E/TRAIL could inhibit tumor growth in a blue light-dependent manner.
Conclusion In this paper, upconversion optogenetic engineered bacteria system was developed for time-resolved imaging diagnosis and light-controlled cancer therapy. The system had the characteristics of accurate diagnosis and controllable treatment. Both EcN@UCNP(s) and FA@UCNP target tumor tissue and the colocation accurately indicated the existence and location of tumor by TRI. In addition, EcN-pDawn-φX174E/TRAIL accumulated at tumor site were induced by blue light to lyse and release TRAIL, thus inducing tumor cell death. We expect that this engineered bacteria system provides a new technology for intelligent bacterial therapy and the construction of lymphoma theranostics.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal